Clinical Protocol and Informed Consent Form Development.
Accelerated with AI.
Software designed to create a structured, guided and more efficient writing experience.
Solutions for different organization types
- Solutions for Institutions, Biotech/Pharma and GME Programs
- Customize and configure software to your process needs
- Enterprise-strength cloud-hosted platform
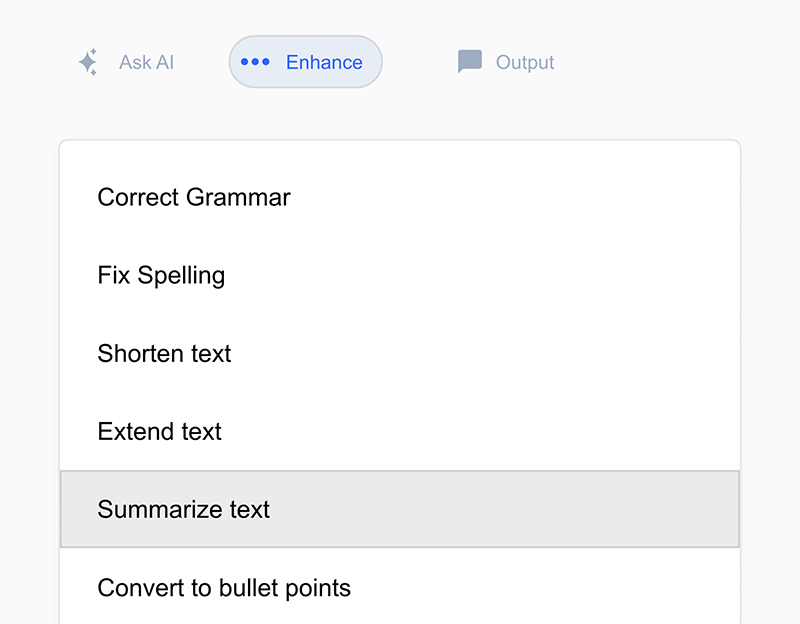
New AI Assistant* and time-saving writing tools
- AI Assistant built into writing experience with familiar format
- Auto populate sample text for entire protocol
- Generate Informed Consent Form draft from protocol and consent form template
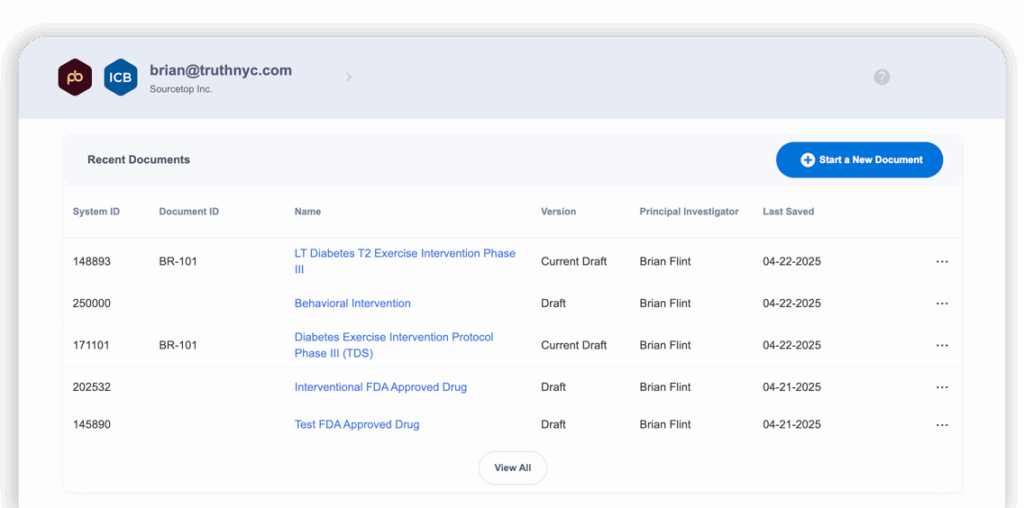
- New document dashboard with foldering and search capabilities
*Premium feature; setup required
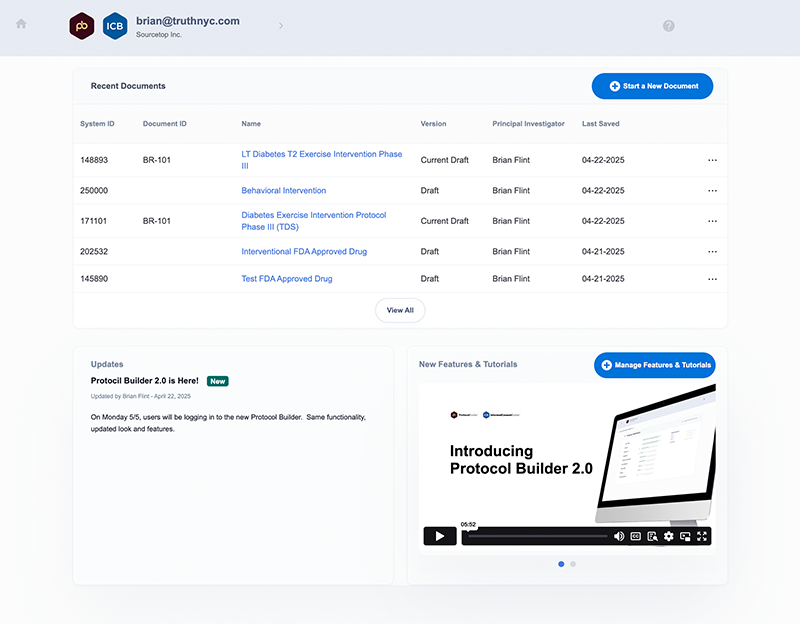
Turn complex protocol writing into a guided, efficient process
- Guided experience keeps investigators on track
- Built-in compliance at every step
- 16 protocol templates included plus template customization for institution-specific needs
- Template cohesion produces more predictable, compliant protocols
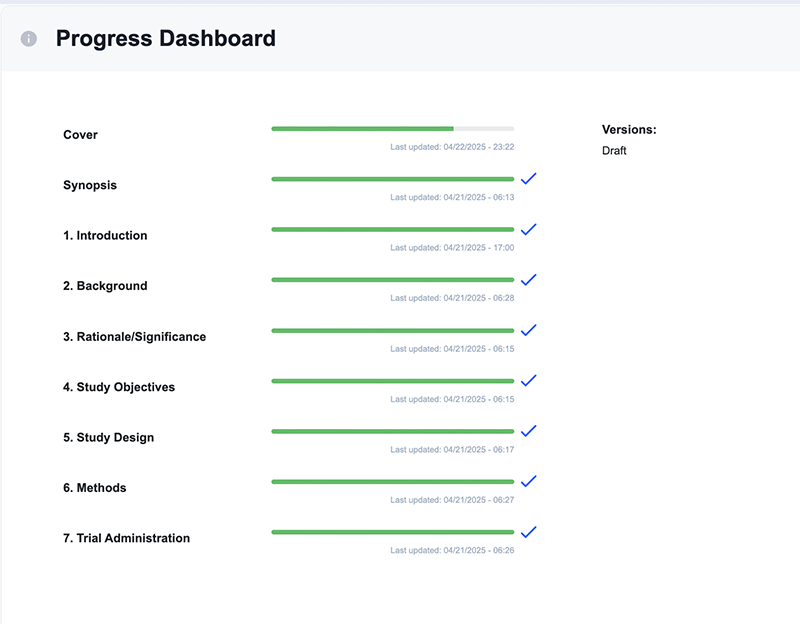
Reduce review delays with higher completion rates and compliance quality
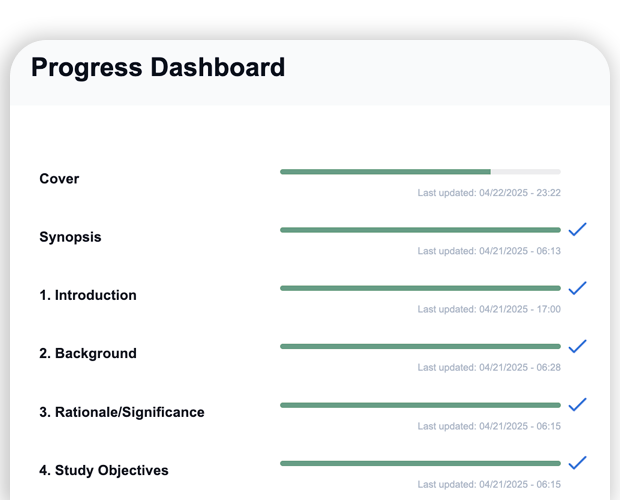
- Progress dashboard reduces submission of incomplete protocols
- Tool tips guidance and sample text drives compliant writing
- Export options to fit IRB needs: Word or PDF, clean copy or with tracked changes
- Eliminate delays due version control issues

Automate Time-Consuming Tasks
- Sample Text: insert sample text by section or for entire protocols
- References Section: automatically numbers and orders references
- List of Table, Charts, Abbreviations and Glossary: automatically
- Consistent, professional formatting—no extra re-formatting effort
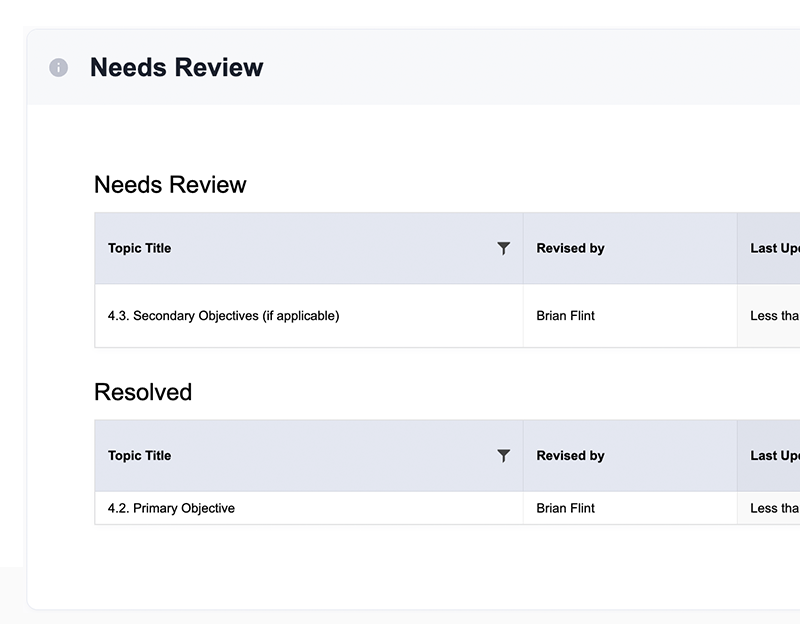
Collaborate seamlessly and eliminate version control issues
- Eliminate version control challenges with clear versioning
- Real-time alerts and notifications
- Select collaborators and access permissions
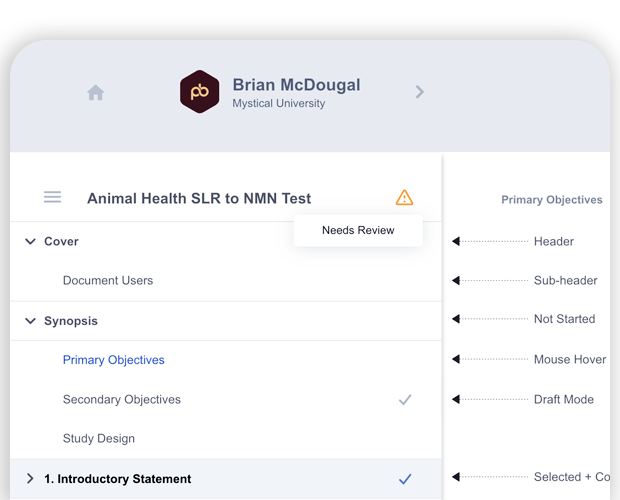
- “Needs Review” worklist highlights sections with changes
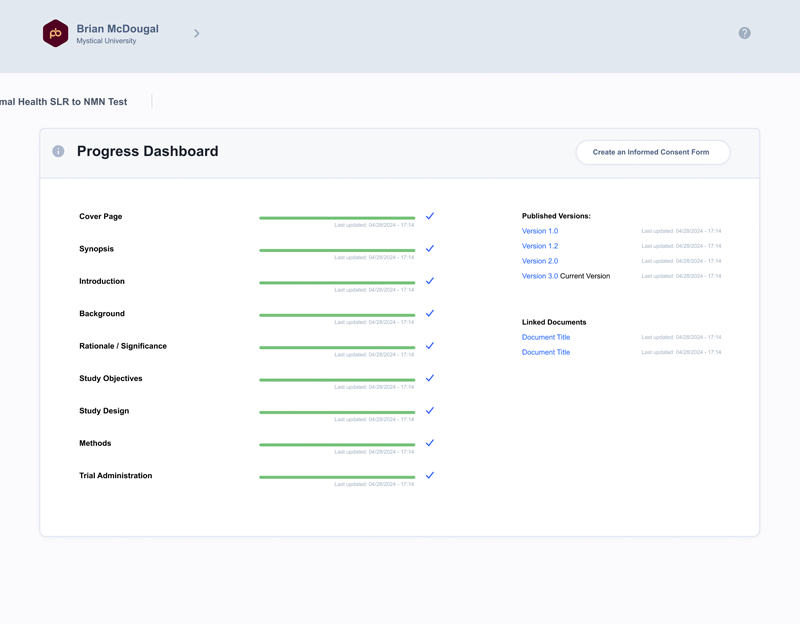
Informed Consent Builder – now with automatic draft generation
- Easier way to build, manage and generate compliant informed consent forms
- Instantly build consent form draft using protocol and informed consent form template language
- Built-in AI Assistant rewrites in 2nd person at 8th-grade level
Onboarding and on-going user support
- New user trainings and on demand one-on-one training
- Help Center with online tutorials
- Help Desk for users and administrators
Admin Control for Compliance and Customization
- Single sign-on setup
- Customize protocol and consent templates
- Tailor guidance, required text, and sample content
- User permissions and custom help center support